Beyond-Li for Stationary Electrochemical Energy Storage
The transition to renewable energy is challenged by the intermittent nature of wind and solar resources. Shifting supply from times of high generation to times of high demand requires energy storage solutions that can be widely deployed at exceptionally low cost. To enable a fully renewable grid, > 300 TWh of energy storage technologies must be installed worldwide at a targeted systems level cost of < 50 $/kWh for medium duration (8-24 h) and <10 $/kWh for long duration (>24 h). Current Li-ion production is on the order of ~0.5-1 TWh/year and expected to grow to ~6 TWh/year by 2030, but almost all of this production is reserved for electric vehicles and even the most ambitious models forecast Li-ion pack prices at ~100 $/kWh in 2050. Thus, alternative "beyond-Li" chemistries that utilize only the most abundant resources must be developed.
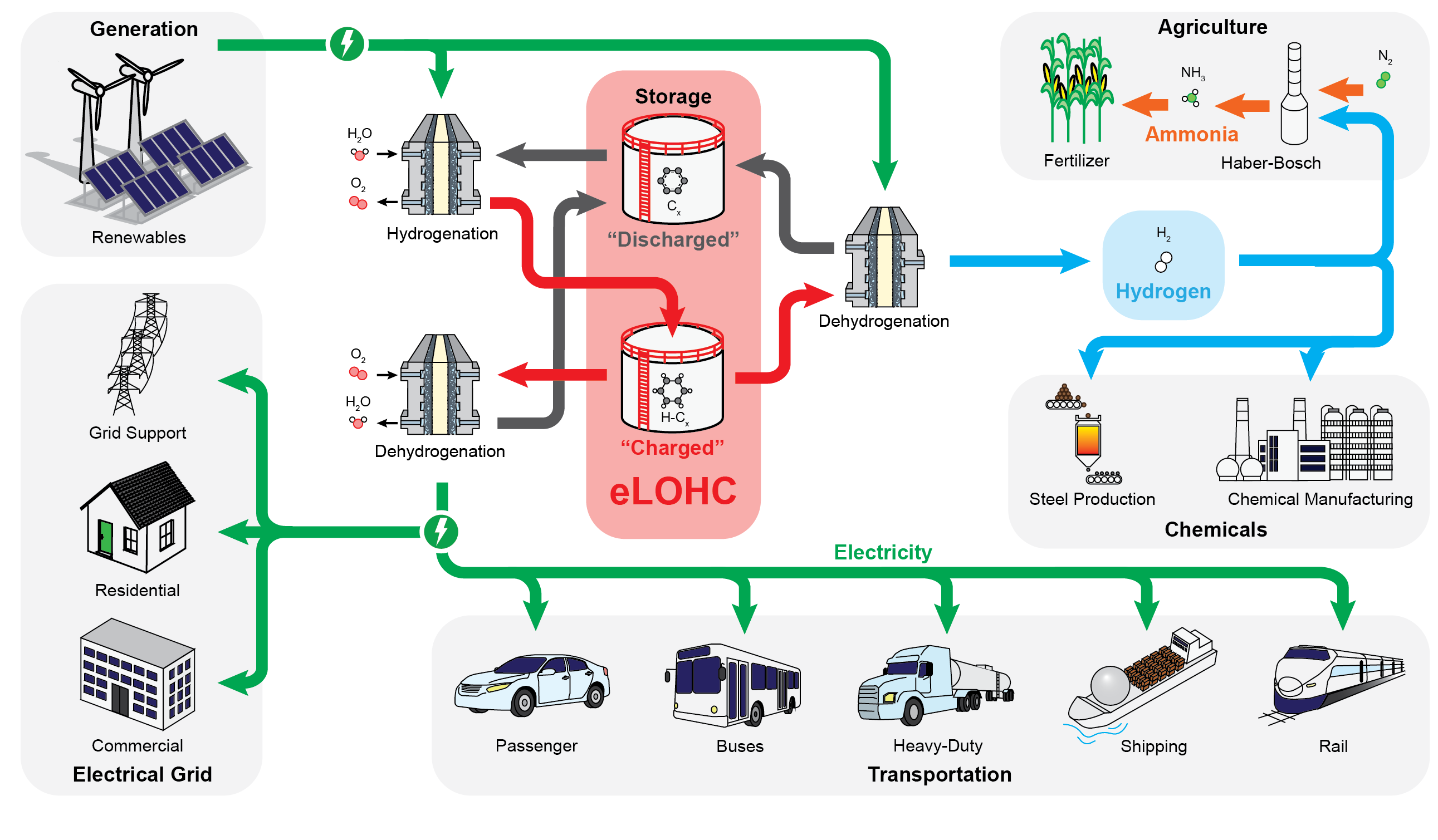
We are developing electrochemical energy storage technologies that utilize "air" cathodes (those that reversibly catalyze the conversion between O2 and H2O) paired with either metal anodes (like Fe, Zn, or Sn) or electrochemical liquid organic hydrogen carriers (eLOHCs) with a targeted chemical storage cost of < 10 $/kWh. eLOHCs are of particular interest as they can help enable the transportation and storage of green hydrogen allowing for simultaneous reduction emissions in energy generation, chemical production, and heavy industry.
Organic Mixed Ionic-Electronic Conducting Polymer Electrocatalysts
Organic mixed ionic-electronic conductors (OMIECs) are a class of conjugated polymers with tunable electronic and ionic transport properties enabled through polaron-forming ion insertion redox reactions. The energy to form these conductive polaronic states can be controlled through rational design of the polymer backbone to enable predominantly electron/cation (n-type) or hole/anion (p-type) transport. Simultaneously, electrolyte uptake into the bulk of the electrode can be controlled through incorporation of polar/non-polar sidechains. The ability to tune the energy of the redox-active states, the majority charge carrier, and the local reaction environment offers an opportunity to independently optimize activity and selectivity in electrochemical energy conversion processes with a single-phase electrode.
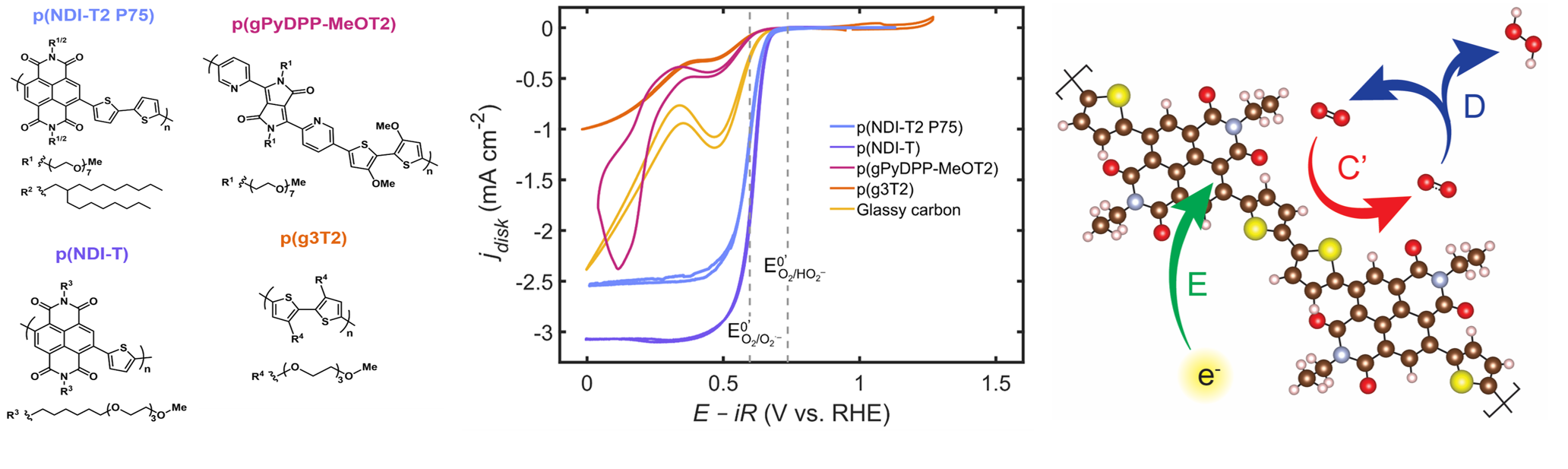
The Mefford group is developing these polymers as metal-free, single-phase electrocatalysts for reactions relevant to energy storage and chemical production, with recent thrusts looking at n-type polymers for the oxygen reduction reaction. We investigate the electronic and chemical origins of reactivity through pH-dependent electrochemistry, operando optical and vibrational spectroscopy, charge-transport measurements, and ab initio/microkinetic simulations. The interplay between chemical structure, electron localization, and charge compensation provide a generalized framework to understand pathway selectivity towards the 2-electron H2O2 or 4-electron H2O product and serve as design principles in developing this emerging class of metal-free electrocatalysts.
Operando Characterization of Electron and Ion Transfer Across Length and Time Scales
Electrochemical systems evolve dynamically and heterogeneously during operation, whether it is through bulk ion-insertion reactions that enable charge storage in technologies like Li-ion batteries, surface redox reactions that form activated sites to drive electrocatalysis, or corrosion processes that lead to electrode dissolution and de-activation. Understanding how electrode composition and structures evolve from the atomic to the mesoscale across timescales helps inform the rationale design of higher performance materials across electrochemical applications.
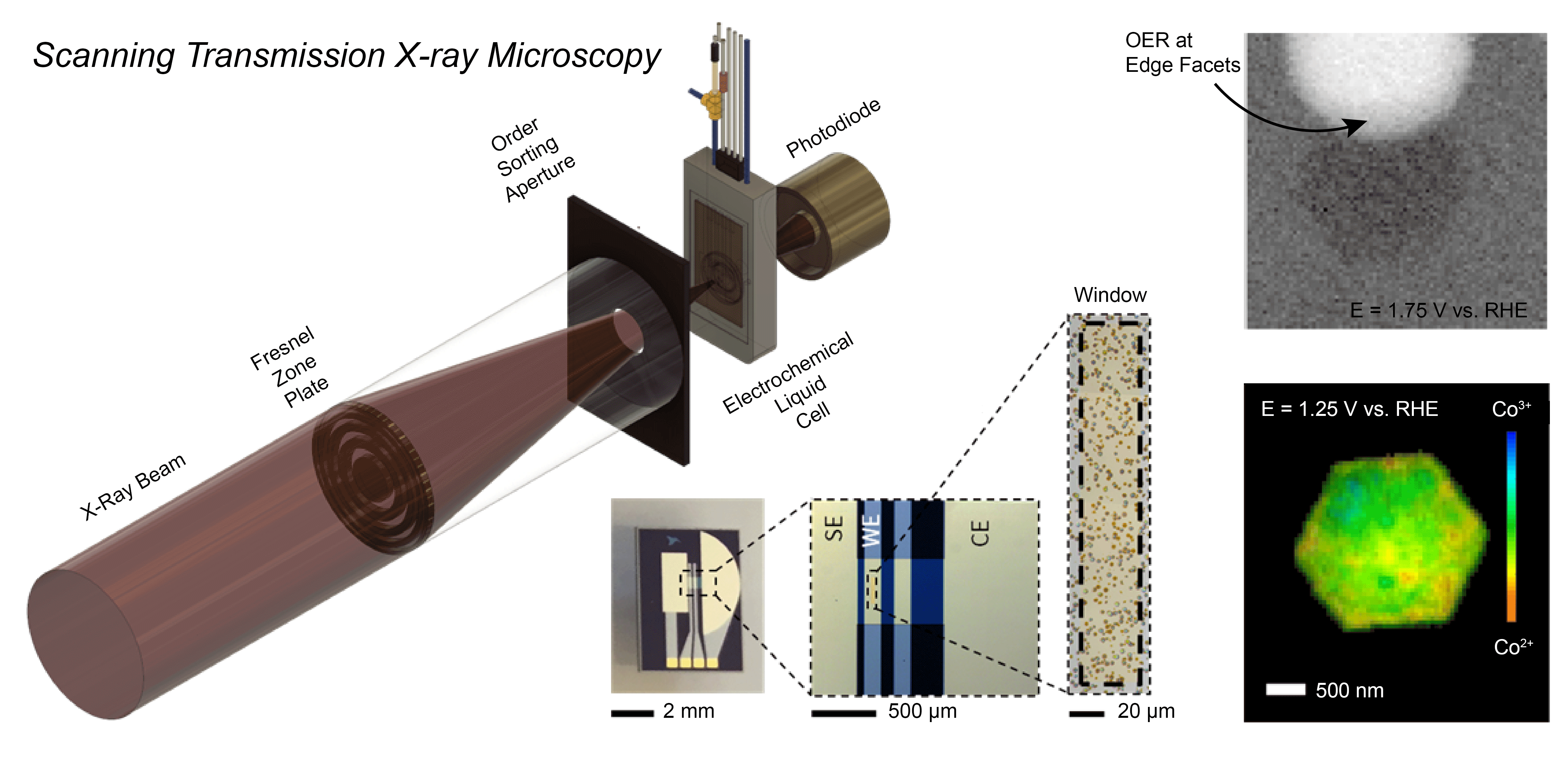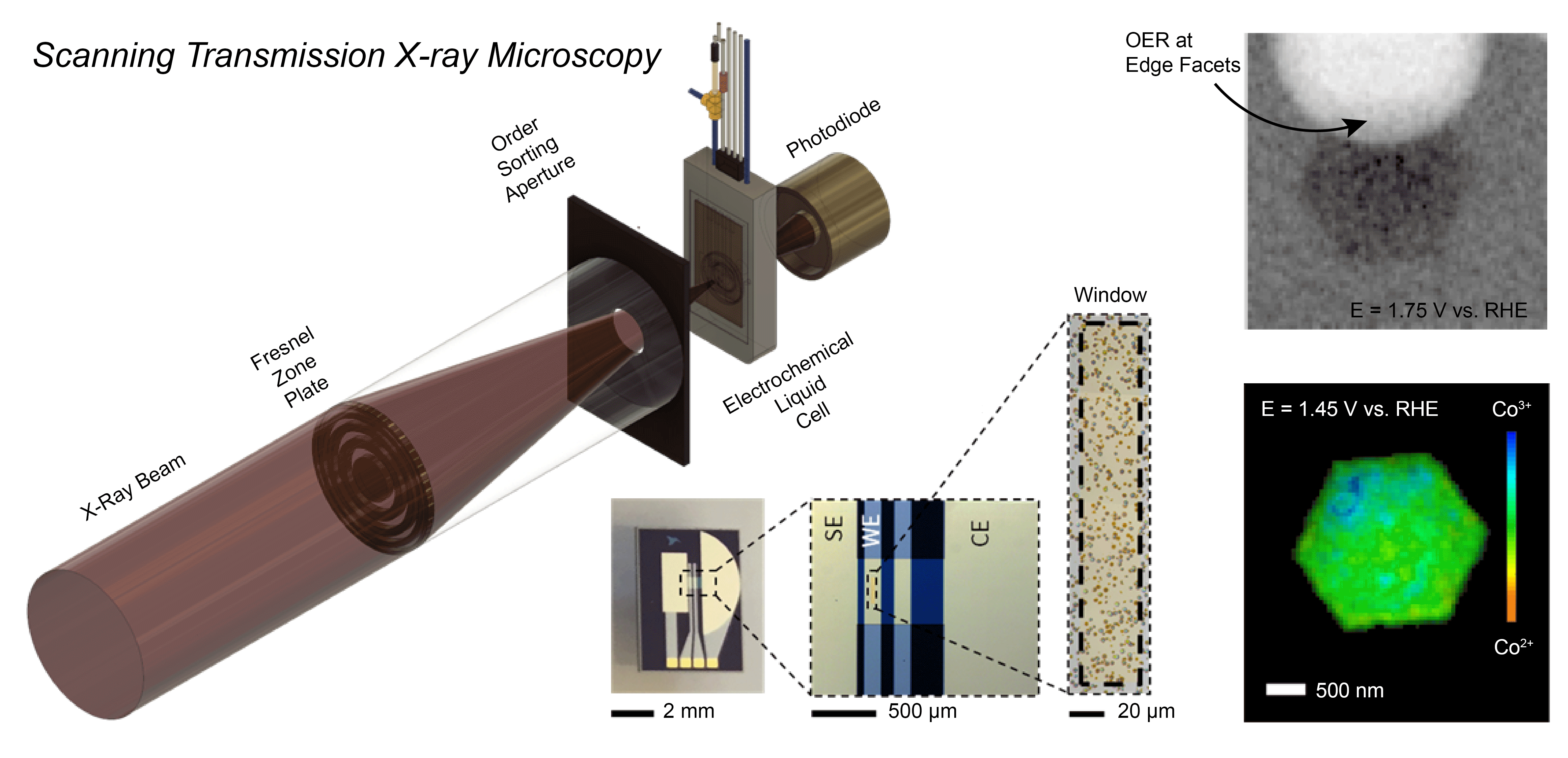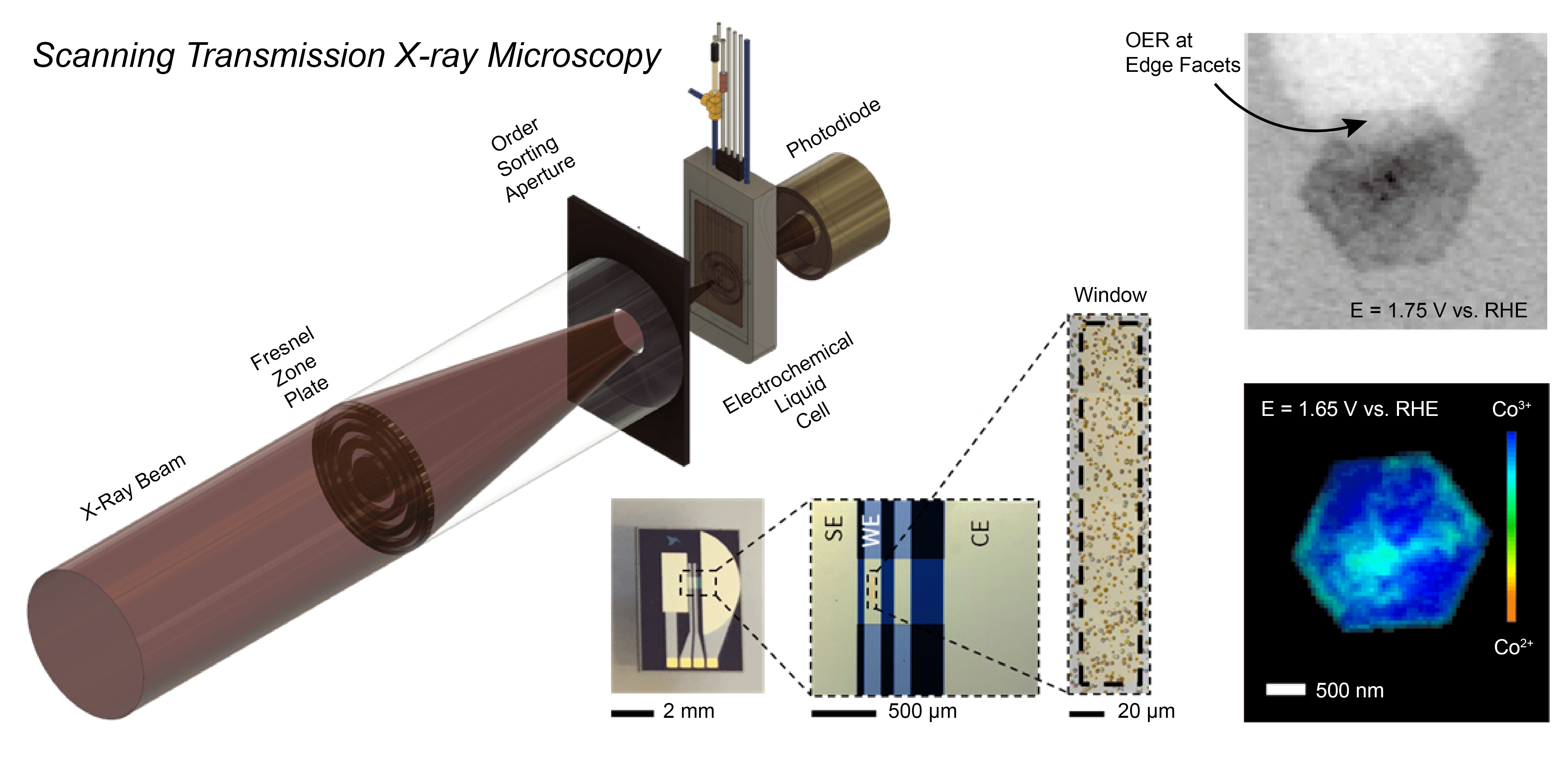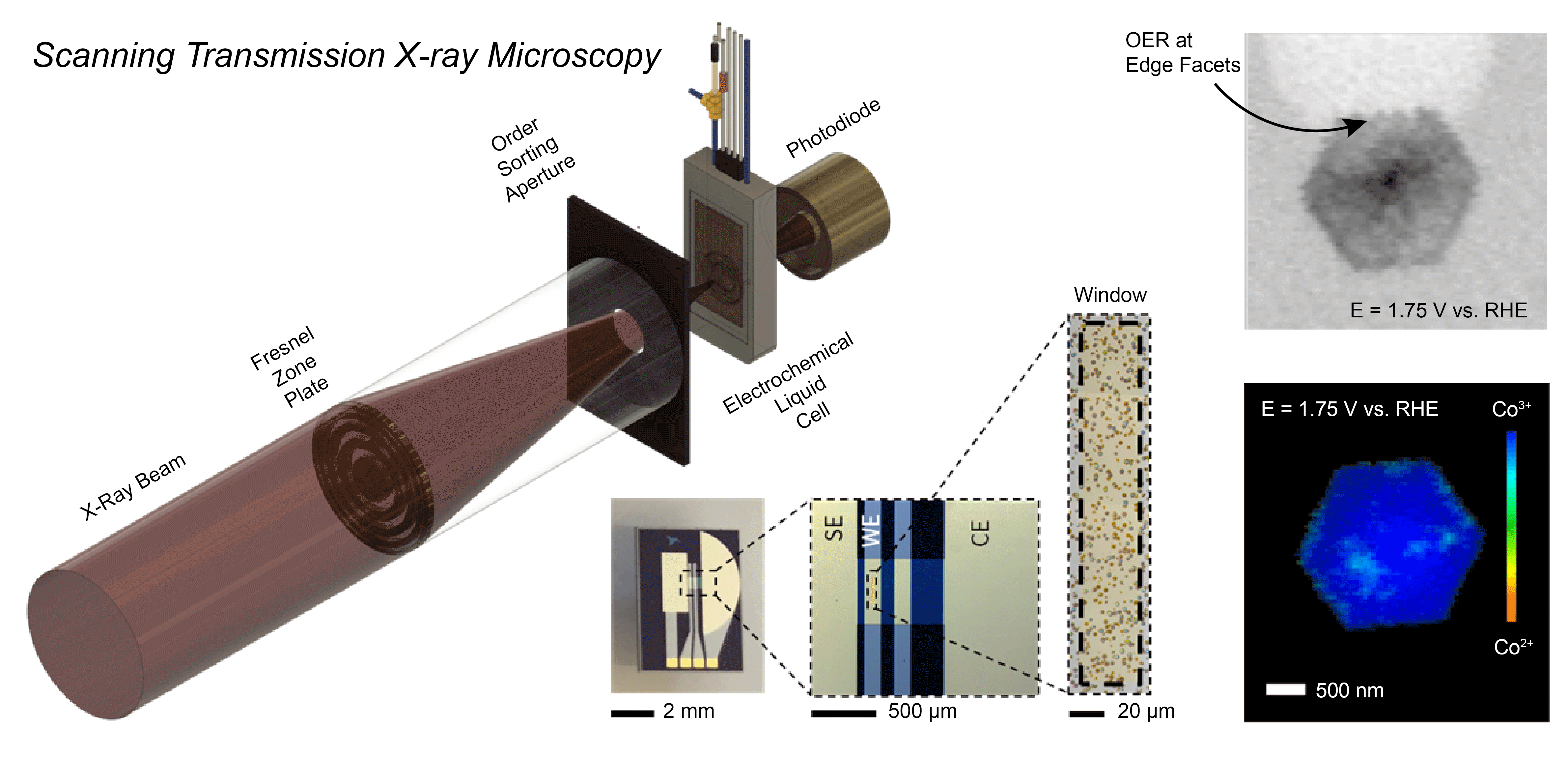
The Mefford Group is focused on developing and utilizing operando characterization across all of our projects. We utilize numerous spectroscopies (XANES/EXAFS, UV-Vis-NIR, ATR-SEIRAS, and Raman), microscopies (STXM, EC-AFM, SECCM), scattering (XRD, GIWAXS), and Differential Electrochemical Mass Spectrometry (DEMS) to characterize the operational chemistry of electrochemical energy storage and conversion materials. In a recent example, we developed a Scanning Transmission X-ray Microscopy (STXM) method to probe where and how CoOxHy particles catalyze the oxygen evolution reaction (OER) at the sub-particle level. During operation, the edges of well-faceted hexagonal particles were found to be the active sites for the OER with the activity correlated to the amount of Co3+ present.